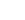N + NO
Gaz phase reaction (type: Bimolecular reactions)
Datasheet T(K) = 10-300
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
10-300 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2013-10-16 |
N + NO → O + N2
T(K): 10-300 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2013-10-16 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
10-300 | 1.07e-11 | 4.00E-11 | -2.00E-1 | 2.00E+1 | 1.4 | 10 | lognormal |
N + NO → O + N2
T(K) = 10-300 α = 4.00E-11 β = -2.00E-1 γ = 2.00E+1 F 0 = 1.4 g = 10 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||
Wennberg, P. O. et al (1994) T(K) = 213-369
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
213-369 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2016-01-06 |
N + NO → O + N2
T(K): 213-369 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2016-01-06 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
213-369 | 1.95e-4 | 2.20E-11 | 0.00E+0 | -1.60E+2 | 0 | 0 | lognormal |
N + NO → O + N2
T(K) = 213-369 α = 2.20E-11 β = 0.00E+0 γ = -1.60E+2 F 0 = 0 g = 0 Type uncert: lognormal Method: Measurements Description: Flow reactor. Microwave discharge of trace N2 in He and atomic resonance fluorescence using a gas filter scheme. |
|
Method: Measurements
Description: Flow reactor. Microwave discharge of trace N2 in He and atomic resonance fluorescence using a gas filter scheme. |
|||||||||
Lee, J. H. et al (1978) T(K) = 196-370
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
196-370 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2025-07-30 |
N + NO → O + N2
T(K): 196-370 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2025-07-30 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
196-370 | 2.70e-11 | 2.70E-11 | 0.00E+0 | 0.00E+0 | 0 | 0 | lognormal |
N + NO → O + N2
T(K) = 196-370 α = 2.70E-11 β = 0.00E+0 γ = 0.00E+0 F 0 = 0 g = 0 Type uncert: lognormal Method: Measurements Description: Discharge flow-filtered resonance fluorescence (DF-RF) and flash photolysis of N2O-resonance fluorescence (FP-RF). |
|
Method: Measurements
Description: Discharge flow-filtered resonance fluorescence (DF-RF) and flash photolysis of N2O-resonance fluorescence (FP-RF). |
|||||||||
Gamallo, P. et al (2006) T(K) = 200-2500
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
200-2500 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2025-07-30 |
N + NO → O + N2
T(K): 200-2500 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2025-07-30 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
200-2500 | 3.94e-6 | 2.15E-11 | 2.80E-1 | -1.31E+2 | 0 | 0 | lognormal |
N + NO → O + N2
T(K) = 200-2500 α = 2.15E-11 β = 2.80E-1 γ = -1.31E+2 F 0 = 0 g = 0 Type uncert: lognormal Method: Calculations Description: Time-dependent real wave-packet (WP) quantum dynamics rate constants on the 1 3A” and 1 3A’ analytical potential energy surfaces (PES). The 3A” PES is barrierless along the minimum energy path, while the analytical 3A’ excited PES presents an energy barrier of 36.57 kJ mol-1, including zero point energy. |
|
Method: Calculations
Description: Time-dependent real wave-packet (WP) quantum dynamics rate constants on the 1 3A” and 1 3A’ analytical potential energy surfaces (PES). The 3A” PES is barrierless along the minimum energy path, while the analytical 3A’ excited PES presents an energy barrier of 36.57 kJ mol-1, including zero point energy. |
|||||||||
Other database : UdFA T(K) = 100-4000
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
100-4000 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2025-07-30 |
N + NO → O + N2
T(K): 100-4000 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2025-07-30 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
100-4000 | 2.79e-12 | 3.75E-11 | 0.00E+0 | 2.60E+1 | 0 | 0 | lognormal |
N + NO → O + N2
T(K) = 100-4000 α = 3.75E-11 β = 0.00E+0 γ = 2.60E+1 F 0 = 0 g = 0 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : UdFA | |||||||
|
Origin: Other database : UdFA
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||
Bergeat, A. et al (2009) T(K) = 48-211
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
48-211 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2009-09-29 |
N + NO → O + N2
T(K): 48-211 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2009-09-29 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
48-211 | 2.63e-12 | 3.20E-11 | 0.00E+0 | 2.50E+1 | 0 | 0 | lognormal |
N + NO → O + N2
T(K) = 48-211 α = 3.20E-11 β = 0.00E+0 γ = 2.50E+1 F 0 = 0 g = 0 Type uncert: lognormal Method: Measurements Description: CRESU N produced by microwave discharge and probed by VUV fluorescence |
|
Method: Measurements
Description: CRESU N produced by microwave discharge and probed by VUV fluorescence |
|||||||||
Baulch, D. L. et al (2005) T(K) = 210-3700
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
210-3700 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2012-08-02 |
N + NO → O + N2
T(K): 210-3700 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2012-08-02 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
210-3700 | 3.50e-11 | 3.50E-11 | 0.00E+0 | 0.00E+0 | 1.26 | 0 | lognormal |
N + NO → O + N2
T(K) = 210-3700 α = 3.50E-11 β = 0.00E+0 γ = 0.00E+0 F 0 = 1.26 g = 0 Type uncert: lognormal Method: Reviews and Evaluations Description: Evaluation of literature data up to 1996. |
|
Method: Reviews and Evaluations
Description: Evaluation of literature data up to 1996. |
|||||||||
Other database : OSU T(K) = 10-280
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2009-03-27 |
N + NO → O + N2
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2009-03-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
10-280 | 2.31e-10 | 3.00E-11 | -6.00E-1 | 0.00E+0 | 2 | 0 | lognormal |
N + NO → O + N2
T(K) = 10-280 α = 3.00E-11 β = -6.00E-1 γ = 0.00E+0 F 0 = 2 g = 0 Type uncert: lognormal Method: Calculations |
|
Method: Calculations
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : OSU | |||||||
|
Origin: Other database : OSU
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||
Duff, J. W. et al (1996) T(K) = 100-1000
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
100-1000 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2025-07-30 |
N + NO → O + N2
T(K): 100-1000 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2025-07-30 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
100-1000 | 2.85e-12 | 3.40E-11 | 0.00E+0 | 2.48E+1 | 0 | 0 | lognormal |
N + NO → O + N2
T(K) = 100-1000 α = 3.40E-11 β = 0.00E+0 γ = 2.48E+1 F 0 = 0 g = 0 Type uncert: lognormal Method: Calculations Description: Quasiclassical trajectory calculations on the 3A” surface of Walch, S. P.; Jaffe, R. L. The Journal of Chemical Physics 1987, 86, 6946-6956, which presents a small energy barrier (in the uncertainty). |
|
Method: Calculations
Description: Quasiclassical trajectory calculations on the 3A” surface of Walch, S. P.; Jaffe, R. L. The Journal of Chemical Physics 1987, 86, 6946-6956, which presents a small energy barrier (in the uncertainty). |
|||||||||
Sander, S. P. et al (2006) T(K) = 298-298
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
298-298 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2025-07-30 |
N + NO → O + N2
T(K): 298-298 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2025-07-30 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
298-298 | 4.63e-7 | 2.10E-11 | 0.00E+0 | -1.00E+2 | 2 | 0 | lognormal |
N + NO → O + N2
T(K) = 298-298 α = 2.10E-11 β = 0.00E+0 γ = -1.00E+2 F 0 = 2 g = 0 Type uncert: lognormal Method: Calculations |
|
Method: Calculations
|
|||||||||
Sander, S.P. et al (2011) T(K) = 200-500
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
200-500 | Modified Arrhenius equation |
ΔrH0 = -317.161
Δ rH298 = -314.776 |
2012-08-01 |
N + NO → O + N2
T(K): 200-500 Formula: Modified Arrhenius equation Δ rH0 = -317.161 Δ rH298 = -314.776 2012-08-01 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NO
→
O + N2
|
200-500 | 4.63e-7 | 2.10E-11 | 0.00E+0 | -1.00E+2 | 1.3 | 100 | lognormal |
N + NO → O + N2
T(K) = 200-500 α = 2.10E-11 β = 0.00E+0 γ = -1.00E+2 F 0 = 1.3 g = 100 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||