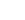HNO + O
Gaz phase reaction (type: Bimolecular reactions)
Datasheet T(K) = 10-298
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
HNO + O
→
NO + OH
|
10-298 | Modified Arrhenius equation |
ΔrH0 = -228.15
Δ rH298 = -227.446 |
2009-10-16 |
HNO + O → NO + OH
T(K): 10-298 Formula: Modified Arrhenius equation Δ rH0 = -228.15 Δ rH298 = -227.446 2009-10-16 |
|
HNO + O
→
H + NO2
|
10-298 | Modified Arrhenius equation |
ΔrH0 = -102.983
Δ rH298 = -103.826 |
2009-10-16 |
HNO + O → H + NO2
T(K): 10-298 Formula: Modified Arrhenius equation Δ rH0 = -102.983 Δ rH298 = -103.826 2009-10-16 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
HNO + O
→
NO + OH
|
10-298 | 4.95e-11 | 3.77E-11 | -8.00E-2 | 0.00E+0 | 2 | 7 | lognormal |
HNO + O → NO + OH
T(K) = 10-298 α = 3.77E-11 β = -8.00E-2 γ = 0.00E+0 F 0 = 2 g = 7 Type uncert: lognormal Method: Reviews and Evaluations Description: See datasheet - result of the ISSI review |
|
Method: Reviews and Evaluations
Description: See datasheet - result of the ISSI review |
|||||||||
|
HNO + O
→
H + NO2
|
10-298 | 0.00e+0 | 0.00E+0 | 0.00E+0 | 0.00E+0 | 0 | 0 | lognormal |
HNO + O → H + NO2
T(K) = 10-298 α = 0.00E+0 β = 0.00E+0 γ = 0.00E+0 F 0 = 0 g = 0 Type uncert: lognormal Method: Reviews and Evaluations Description: See datasheet - result of the ISSI review |
|
Method: Reviews and Evaluations
Description: See datasheet - result of the ISSI review |
|||||||||
Other database : UdFA T(K) = 10-800
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
HNO + O
→
NH + O2
|
10-800 | Modified Arrhenius equation |
ΔrH0 = 2.743
Δ rH298 = 2.775 |
2014-03-05 |
HNO + O → NH + O2
T(K): 10-800 Formula: Modified Arrhenius equation Δ rH0 = 2.743 Δ rH298 = 2.775 2014-03-05 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
HNO + O
→
NH + O2
|
10-800 | 0.00e+0 | 2.94E-12 | 5.00E-1 | 3.50E+3 | 2 | 0 | lognormal |
HNO + O → NH + O2
T(K) = 10-800 α = 2.94E-12 β = 5.00E-1 γ = 3.50E+3 F 0 = 2 g = 0 Type uncert: lognormal Method: Estimation Description: Reactions used in the high temperature network for ISM applications by Harada et al. (2010). Value not listed in the NIST. |
|
Method: Estimation
Description: Reactions used in the high temperature network for ISM applications by Harada et al. (2010). Value not listed in the NIST. |
|||||||||
Other database : OSU T(K) = 10-280
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
HNO + O
→
NO + OH
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -228.15
Δ rH298 = -227.446 |
2009-03-27 |
HNO + O → NO + OH
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -228.15 Δ rH298 = -227.446 2009-03-27 |
|
HNO + O
→
H + NO2
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -102.983
Δ rH298 = -103.826 |
2009-03-27 |
HNO + O → H + NO2
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -102.983 Δ rH298 = -103.826 2009-03-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
HNO + O
→
NO + OH
|
10-280 | 3.80e-11 | 3.80E-11 | 0.00E+0 | 0.00E+0 | 1.25 | 0 | lognormal |
HNO + O → NO + OH
T(K) = 10-280 α = 3.80E-11 β = 0.00E+0 γ = 0.00E+0 F 0 = 1.25 g = 0 Type uncert: lognormal Method: Estimation |
|
Method: Estimation
|
|||||||||
|
HNO + O
→
H + NO2
|
10-280 | 1.00e-12 | 1.00E-12 | 0.00E+0 | 0.00E+0 | 2 | 0 | lognormal |
HNO + O → H + NO2
T(K) = 10-280 α = 1.00E-12 β = 0.00E+0 γ = 0.00E+0 F 0 = 2 g = 0 Type uncert: lognormal Method: Estimation |
|
Method: Estimation
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : OSU | |||||||
|
Origin: Other database : OSU
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||