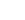C+ + S
Gaz phase reaction (type: Charge exchange reactions)
Chenel, A. et al (2010) T(K) = 500-50000
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
C+ + S
→
C + S+
|
500-50000 | Modified Arrhenius equation |
ΔrH0 = -100.902
Δ rH298 = -87.448 |
2009-10-23 |
C+ + S → C + S+
T(K): 500-50000 Formula: Modified Arrhenius equation Δ rH0 = -100.902 Δ rH298 = -87.448 2009-10-23 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
C+ + S
→
C + S+
|
500-50000 | 0.00e+0 | 5.54E-12 | 8.58E-1 | 6.81E+2 | 1.5 | 0 | lognormal |
C+ + S → C + S+
T(K) = 500-50000 α = 5.54E-12 β = 8.58E-1 γ = 6.81E+2 F 0 = 1.5 g = 0 Type uncert: lognormal Method: Calculations Description: Rate constante calculations have been calculated from accurate ab intio surfaces and semi classical dynamics. From these rate constantes the alpha, beta and gamma coefficients of the Kooij formulae have been deduced |
|
Method: Calculations
Description: Rate constante calculations have been calculated from accurate ab intio surfaces and semi classical dynamics. From these rate constantes the alpha, beta and gamma coefficients of the Kooij formulae have been deduced |
|||||||||
Other database : OSU T(K) = 10-280
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
C+ + S
→
C + S+
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -100.902
Δ rH298 = -87.448 |
2009-03-27 |
C+ + S → C + S+
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -100.902 Δ rH298 = -87.448 2009-03-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
C+ + S
→
C + S+
|
10-280 | 1.50e-9 | 1.50E-9 | 0.00E+0 | 0.00E+0 | 2 | 0 | lognormal |
C+ + S → C + S+
T(K) = 10-280 α = 1.50E-9 β = 0.00E+0 γ = 0.00E+0 F 0 = 2 g = 0 Type uncert: lognormal Method: Estimation |
|
Method: Estimation
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : OSU | |||||||
|
Origin: Other database : OSU
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||
Vidal, T.~H.~G. et al (2017) T(K) = 10-800
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
C+ + S
→
C + S+
|
10-800 | Modified Arrhenius equation |
ΔrH0 = -100.902
Δ rH298 = -87.448 |
2017-07-19 |
C+ + S → C + S+
T(K): 10-800 Formula: Modified Arrhenius equation Δ rH0 = -100.902 Δ rH298 = -87.448 2017-07-19 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
C+ + S
→
C + S+
|
10-800 | 1.30e-9 | 1.30E-9 | 0.00E+0 | 0.00E+0 | 10 | 0 | logn |
C+ + S → C + S+
T(K) = 10-800 α = 1.30E-9 β = 0.00E+0 γ = 0.00E+0 F 0 = 10 g = 0 Type uncert: logn Method: Reviews and Evaluations Description: See KIDA datasheet. Large uncertainty. |
|
Method: Reviews and Evaluations
Description: See KIDA datasheet. Large uncertainty. |
|||||||||