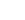CH3OH + H+
Gaz phase reaction (type: Charge exchange reactions)
Woon, D. E. et al (2009) T(K) = 10-800
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
CH3OH + H+
→
H + CH3OH+
|
10-800 | ionpol1 |
ΔrH0 = -262.29
Δ rH298 = -264.813 |
2011-10-14 |
CH3OH + H+ → H + CH3OH+
T(K): 10-800 Formula: ionpol1 Δ rH0 = -262.29 Δ rH298 = -264.813 2011-10-14 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
CH3OH + H+
→
H + CH3OH+
|
10-800 | 1.95e-8 | 5.00E-1 | 4.19E-9 | 3.32E+0 | 2 | 0 | lognormal |
CH3OH + H+ → H + CH3OH+
T(K) = 10-800 α = 5.00E-1 β = 4.19E-9 γ = 3.32E+0 F 0 = 2 g = 0 Type uncert: lognormal Method: Calculations Description: Rate coefficients for ion-polar reactions are computed by two different formula depending on the temperature. Note that the formula are different from the Arrhenius-Kooij form used for the other bimolecular reactions. Alpha represents the branching ratio of the channel and beta the langevin rate. Some details can also be found in Wakelam et al. (2010, Space Science Reviews, 156, 13) |
|
Method: Calculations
Description: Rate coefficients for ion-polar reactions are computed by two different formula depending on the temperature. Note that the formula are different from the Arrhenius-Kooij form used for the other bimolecular reactions. Alpha represents the branching ratio of the channel and beta the langevin rate. Some details can also be found in Wakelam et al. (2010, Space Science Reviews, 156, 13) |
|||||||||
Other database : OSU T(K) = 10-280
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
CH3OH + H+
→
H + CH3OH+
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -262.29
Δ rH298 = -264.813 |
2009-03-27 |
CH3OH + H+ → H + CH3OH+
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -262.29 Δ rH298 = -264.813 2009-03-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
CH3OH + H+
→
H + CH3OH+
|
10-280 | 1.81e-8 | 3.30E-9 | -5.00E-1 | 0.00E+0 | 1.5 | 0 | lognormal |
CH3OH + H+ → H + CH3OH+
T(K) = 10-280 α = 3.30E-9 β = -5.00E-1 γ = 0.00E+0 F 0 = 1.5 g = 0 Type uncert: lognormal Method: Estimation |
|
Method: Estimation
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : OSU | |||||||
|
Origin: Other database : OSU
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||