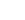OH + OH
Gaz phase reaction (type: Bimolecular reactions)
Atkinson, R. et al (2004) T(K) = 200-350
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
200-350 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2010-11-23 |
OH + OH → O + H2O
T(K): 200-350 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2010-11-23 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
200-350 | 9.19e+23 | 6.20E-14 | 2.62E+0 | -9.45E+2 | 1.2 | 125 | lognormal |
OH + OH → O + H2O
T(K) = 200-350 α = 6.20E-14 β = 2.62E+0 γ = -9.45E+2 F 0 = 1.2 g = 125 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||
Bedjanian, Y et al (1999) T(K) = 233-360
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
233-360 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2010-05-31 |
OH + OH → O + H2O
T(K): 233-360 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2010-05-31 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
233-360 | 9.36e-4 | 7.10E-13 | 0.00E+0 | -2.10E+2 | 1.3 | 0 | lognormal |
OH + OH → O + H2O
T(K) = 233-360 α = 7.10E-13 β = 0.00E+0 γ = -2.10E+2 F 0 = 1.3 g = 0 Type uncert: lognormal Method: Measurements |
|
Method: Measurements
|
|||||||||
Sun, H. et al (2004) T(K) = 220-320
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
220-320 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2010-06-01 |
OH + OH → O + H2O
T(K): 220-320 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2010-06-01 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
220-320 | 6.32e+6 | 2.70E-13 | 0.00E+0 | -4.46E+2 | 1.3 | 0 | lognormal |
OH + OH → O + H2O
T(K) = 220-320 α = 2.70E-13 β = 0.00E+0 γ = -4.46E+2 F 0 = 1.3 g = 0 Type uncert: lognormal Method: Measurements Description: Discharge flow/mass spectrometer/resonance fluorescence method |
|
Method: Measurements
Description: Discharge flow/mass spectrometer/resonance fluorescence method |
|||||||||
Sander, S.P. et al (2011) T(K) = 200-500
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
200-500 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2012-08-01 |
OH + OH → O + H2O
T(K): 200-500 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2012-08-01 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
200-500 | 1.80e-12 | 1.80E-12 | 0.00E+0 | 0.00E+0 | 1.25 | 50 | lognormal |
OH + OH → O + H2O
T(K) = 200-500 α = 1.80E-12 β = 0.00E+0 γ = 0.00E+0 F 0 = 1.25 g = 50 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||
Baulch, D. L. et al (2005) T(K) = 250-2400
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
250-2400 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2012-08-02 |
OH + OH → O + H2O
T(K): 250-2400 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2012-08-02 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
250-2400 | 0.00e+0 | 5.49E-14 | 2.42E+0 | 9.70E+2 | 1.12 | 0 | lognormal |
OH + OH → O + H2O
T(K) = 250-2400 α = 5.49E-14 β = 2.42E+0 γ = 9.70E+2 F 0 = 1.12 g = 0 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||
Other database : OSU T(K) = 10-280
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2009-03-27 |
OH + OH → O + H2O
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2009-03-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
10-280 | 2.30e-16 | 1.65E-12 | 1.14E+0 | 5.00E+1 | 2 | 0 | lognormal |
OH + OH → O + H2O
T(K) = 10-280 α = 1.65E-12 β = 1.14E+0 γ = 5.00E+1 F 0 = 2 g = 0 Type uncert: lognormal Method: Estimation |
|
Method: Estimation
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : OSU | |||||||
|
Origin: Other database : OSU
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||
Hebrard, E. et al (2009) T(K) = 50-200
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
50-200 | Modified Arrhenius equation |
ΔrH0 = -29.432
Δ rH298 = -29.951 |
2012-07-27 |
OH + OH → O + H2O
T(K): 50-200 Formula: Modified Arrhenius equation Δ rH0 = -29.432 Δ rH298 = -29.951 2012-07-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
O + H2O
|
50-200 | 1.23e+24 | 6.34E-14 | 2.60E+0 | -9.47E+2 | 1.4 | 240 | lognormal |
OH + OH → O + H2O
T(K) = 50-200 α = 6.34E-14 β = 2.60E+0 γ = -9.47E+2 F 0 = 1.4 g = 240 Type uncert: lognormal Method: Reviews and Evaluations Description: Taken from Atkinson et al. [2004]. Used in Hébrard et al. [2009] |
|
Method: Reviews and Evaluations
Description: Taken from Atkinson et al. [2004]. Used in Hébrard et al. [2009] |
|||||||||
Other database : NIST T(K) = 10-800
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
OH + OH
→
H + O2H
|
10-800 | Modified Arrhenius equation |
ΔrH0 = 193.942
Δ rH298 = 192.994 |
2014-03-05 |
OH + OH → H + O2H
T(K): 10-800 Formula: Modified Arrhenius equation Δ rH0 = 193.942 Δ rH298 = 192.994 2014-03-05 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
OH + OH
→
H + O2H
|
10-800 | 0.00e+0 | 1.82E-40 | 0.00E+0 | 0.00E+0 | 2 | 0 | lognormal |
OH + OH → H + O2H
T(K) = 10-800 α = 1.82E-40 β = 0.00E+0 γ = 0.00E+0 F 0 = 2 g = 0 Type uncert: lognormal Method: Estimation Description: Reactions used in the high temperature network for ISM applications by Harada et al. (2010). Value listed in the NIST. |
|
Method: Estimation
Description: Reactions used in the high temperature network for ISM applications by Harada et al. (2010). Value listed in the NIST. |
|||||||||