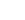O
Click on the icon info to get details.
Species data
| Common Formula | O |
| Stoichiometric Formula | O |
| Name | Oxygen atom |
| Mass | 15.99491 a.m.u |
| Charge | 0 |
| CAS | 17778-80-2 |
| Inchi | InChI=1S/O |
| InchiKey | QVGXLLKOCUKJST-UHFFFAOYSA-N |
| State | Ground State
|
ISM Abundance
| log10 Abundance | Reference | Source Name | Source Type | Link |
|---|
Polarizability
| Evaluation | Definition | Value (Å3) | Method | Origin | Reference |
|---|---|---|---|---|---|
| total | 0.79 ±0.03 | Reviews and Evaluations | Bibliography | Schwerdtfeger, Peter et al. ;2019;Molecular Physics;117, 1200-1225 | |
| total | 0.802 ±0.01604 | Calculations | Bibliography | Lide, D. R.. ;2000; |
|
Definition: total Value (Å3): 0.79 ±0.03 Method: Reviews and Evaluations Origin: Bibliography Reference: Schwerdtfeger, Peter et al. ;2019;Molecular Physics;117, 1200-1225 |
|
Definition: total Value (Å3): 0.802 ±0.01604 Method: Calculations Origin: Bibliography Reference: Lide, D. R.. ;2000; |
Dipole moment
| Evaluation | Value (D) | Method | Origin | Reference |
|---|---|---|---|---|
| 0 | N/A | Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE |
|
Value (D):
0 Method: N/A Origin: Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE Reference: |
Enthalpy of formation
| Evaluation | T (K) | Value (kJ.mol-1) | Method | Origin | Reference |
|---|---|---|---|---|---|
| 298 | 249.175 ±0.002 | Reviews and Evaluations | Database : Burcat | ||
| 0 | 246.79 ±0.002 | Reviews and Evaluations | Database : Burcat | ||
| 0 | 246.84 | Measurements | Database : CCCBDB (http://cccbdb.nist.gov/) | ||
| 298 | 249.23 | Measurements | Database : CCCBDB (http://cccbdb.nist.gov/) |
|
T (K): 298
Value (kJ.mol-1) : 249.175 ±0.002 Method: Reviews and Evaluations Origin: Other database Reference: |
|
T (K): 0
Value (kJ.mol-1) : 246.79 ±0.002 Method: Reviews and Evaluations Origin: Other database Reference: |
|
T (K): 0
Value (kJ.mol-1) : 246.84 Method: Measurements Origin: Other database Reference: |
|
T (K): 298
Value (kJ.mol-1) : 249.23 Method: Measurements Origin: Other database Reference: |
Desorption energy
| Evaluation | Emean (K) | Emin (K) | Emax (K) | Pre-exponential factor (s-1) | Order factor | Method | Origin | Reference | Type of surface | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 1821 | 3.10E+13 | 1 | Reviews and Evaluations | Bibliography | Minissale, Marco et al. ;2022;ACS Earth and Space Chemistry;, | Oxydized Highly-Oriented Pyrolytic Graphite | Uncertainties are discussed in Minissale et al. (2022) | |||
| 1751 | 2.73E+13 | 1 | Reviews and Evaluations | Bibliography | Minissale, Marco et al. ;2022;ACS Earth and Space Chemistry;, | Compact Amorphous Solid Water | Uncertainties are discussed in Minissale et al. (2022) | |||
| 1600 ±480 | 0 | 0 | 0.00E+0 | 1 | Calculations | Bibliography | Wakelam, V. et al. ;2017;ArXiv e-prints;, | H2O | To estimate the unknown binding energies (for most of the radicals for example), we have developed a model founded on the stabilization energy of the complex between the various species and one water molecule. Then, we assume that the binding energy of the species with ASW is proportional to the energy of interaction between this species and one water molecule. To determine the proportionality coefficients, we fit the dependency of the experimental binding energies versus the calculated energies of the complexes for 16 stable molecules. Uncertainties in ED is estimated to be 30%. The preexponential factor is to be computed using the Hasegawa et al. (1992) approximation. | |
| 1850 ±90 | 0 | 0 | 1.00E+12 | 0 | Measurements | Bibliography | He, J. et al. ;2015;Astrophysical Journal;801, 120 | SiOx / Silicate | ||
| 1660 ±60 | 0 | 0 | 1.00E+12 | 0 | Measurements | Bibliography | He, J. et al. ;2015;Astrophysical Journal;801, 120 | H2O / Amorphous / Porous |
|
Emean (K): 1821
E min (K): E max (K): Pre-exponential factor (s-1): 3.10E+13 Method: Reviews and Evaluations Origin: Bibliography Reference: Minissale, Marco et al. ;2022;ACS Earth and Space Chemistry;, Type of surface: Oxydized Highly-Oriented Pyrolytic Graphite Description: Uncertainties are discussed in Minissale et al. (2022) Evaluation: |
|
Emean (K): 1751
E min (K): E max (K): Pre-exponential factor (s-1): 2.73E+13 Method: Reviews and Evaluations Origin: Bibliography Reference: Minissale, Marco et al. ;2022;ACS Earth and Space Chemistry;, Type of surface: Compact Amorphous Solid Water Description: Uncertainties are discussed in Minissale et al. (2022) Evaluation: |
|
Emean (K): 1600 ±480
E min (K): 0 E max (K): 0 Pre-exponential factor (s-1): 0.00E+0 Method: Calculations Origin: Bibliography Reference: Wakelam, V. et al. ;2017;ArXiv e-prints;, Type of surface: H2O Description: To estimate the unknown binding energies (for most of the radicals for example), we have developed a model founded on the stabilization energy of the complex between the various species and one water molecule. Then, we assume that the binding energy of the species with ASW is proportional to the energy of interaction between this species and one water molecule. To determine the proportionality coefficients, we fit the dependency of the experimental binding energies versus the calculated energies of the complexes for 16 stable molecules. Uncertainties in ED is estimated to be 30%. The preexponential factor is to be computed using the Hasegawa et al. (1992) approximation. Evaluation: |
|
Emean (K): 1850 ±90
E min (K): 0 E max (K): 0 Pre-exponential factor (s-1): 1.00E+12 Method: Measurements Origin: Bibliography Reference: He, J. et al. ;2015;Astrophysical Journal;801, 120 Type of surface: SiOx / Silicate Description: Evaluation: |
|
Emean (K): 1660 ±60
E min (K): 0 E max (K): 0 Pre-exponential factor (s-1): 1.00E+12 Method: Measurements Origin: Bibliography Reference: He, J. et al. ;2015;Astrophysical Journal;801, 120 Type of surface: H2O / Amorphous / Porous Description: Evaluation: |
Diffusion energy
No data