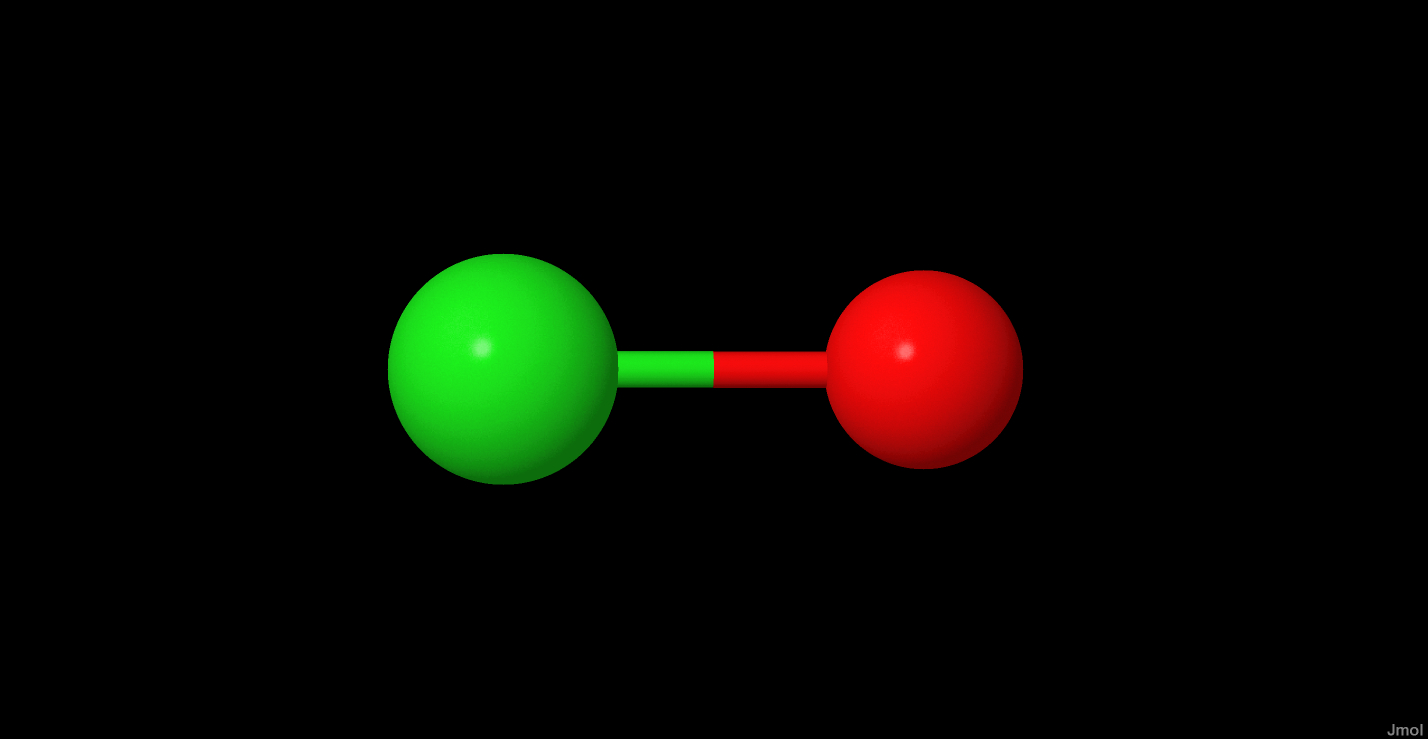
ClO+
Click on the icon info to get details.
Species data
| Common Formula | ClO+ |
| Stoichiometric Formula | ClO+ |
| Name | chlorine monoxide cation |
| Mass | 50.96322 a.m.u |
| Charge | 1 |
| CAS | |
| Inchi | InChI=1S/ClO/c1-2/q+1 |
| InchiKey | GPDKCNQUFLZMCS-UHFFFAOYSA-N |
| State | Ground State
|
ISM Abundance
| log10 Abundance | Reference | Source Name | Source Type | Link |
|---|
Polarizability
| Evaluation | Definition | Value (Å3) | Method | Origin | Reference |
|---|---|---|---|---|---|
| total | 1.812 | Calculations | Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE |
|
Definition: total Value (Å3): 1.812 Method: Calculations Origin: Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE Reference: |
Dipole moment
| Evaluation | Value (D) | Method | Origin | Reference |
|---|---|---|---|---|
| 1.248 | Calculations | Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE |
|
Value (D):
1.248 Method: Calculations Origin: Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE Reference: |
Enthalpy of formation
| Evaluation | T (K) | Value (kJ.mol-1) | Method | Origin | Reference |
|---|---|---|---|---|---|
| 0 | 275.2 ±0.37 | Measurements | Bibliography | Sablier, M. et al. ;2002;Chemical Reviews;102,2855-2924 |
|
T (K): 0
Value (kJ.mol-1) : 275.2 ±0.37 Method: Measurements Origin: Bibliography Reference: Sablier, M. et al. ;2002;Chemical Reviews;102,2855-2924 |
Desorption energy
No data
Diffusion energy
No data