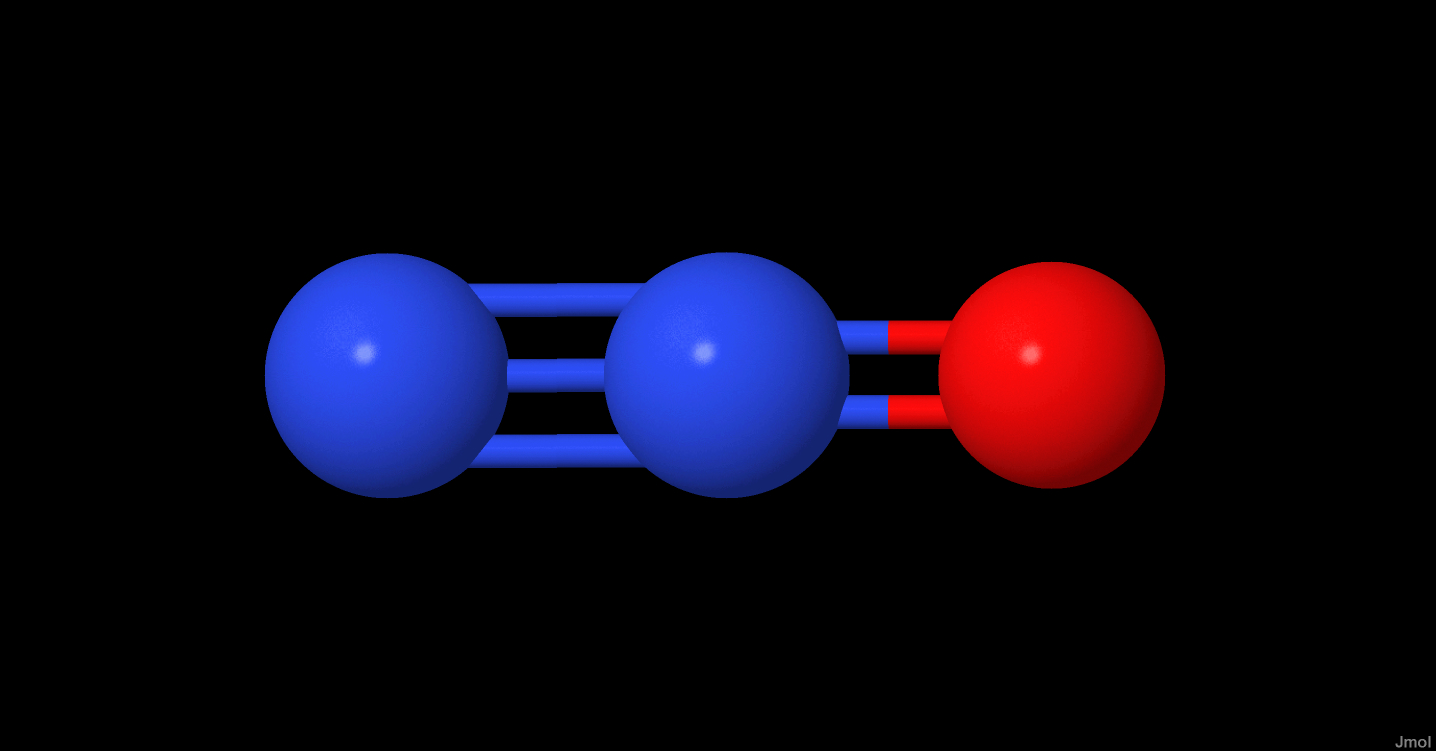
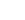N2O
Click on the icon info to get details.
Species data
| Common Formula | N2O |
| Stoichiometric Formula | N2O |
| Name | Nitrous oxide |
| Mass | 44.00106 a.m.u |
| Charge | 0 |
| CAS | 10024-97-2 |
| Inchi | InChI=1S/N2O/c1-2-3 |
| InchiKey | GQPLMRYTRLFLPF-UHFFFAOYSA-N |
| State | Ground State
|
ISM Abundance
| log10 Abundance | Reference | Source Name | Source Type | Link |
|---|
Polarizability
| Evaluation | Definition | Value (Å3) | Method | Origin | Reference |
|---|---|---|---|---|---|
| total | 2.998 | Measurements | Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE |
|
Definition: total Value (Å3): 2.998 Method: Measurements Origin: Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE Reference: |
Dipole moment
| Evaluation | Value (D) | Method | Origin | Reference |
|---|---|---|---|---|
| 0.16083 | Measurements | Bibliography | Lide, D. R.. ;2004; |
|
Value (D):
0.16083 Method: Measurements Origin: Bibliography Reference: Lide, D. R.. ;2004; |
Enthalpy of formation
| Evaluation | T (K) | Value (kJ.mol-1) | Method | Origin | Reference |
|---|---|---|---|---|---|
| 0 | 85.029 ±0.1 | Reviews and Evaluations | Database : Burcat | ||
| 298 | 81.6 ±0.1 | Reviews and Evaluations | Database : Burcat | ||
| 298 | 81.6 ±0.5 | Measurements | Database : CCCBDB (http://cccbdb.nist.gov/) | ||
| 0 | 85.03 ±0.5 | Measurements | Database : CCCBDB (http://cccbdb.nist.gov/) |
|
T (K): 0
Value (kJ.mol-1) : 85.029 ±0.1 Method: Reviews and Evaluations Origin: Other database Reference: |
|
T (K): 298
Value (kJ.mol-1) : 81.6 ±0.1 Method: Reviews and Evaluations Origin: Other database Reference: |
|
T (K): 298
Value (kJ.mol-1) : 81.6 ±0.5 Method: Measurements Origin: Other database Reference: |
|
T (K): 0
Value (kJ.mol-1) : 85.03 ±0.5 Method: Measurements Origin: Other database Reference: |
Desorption energy
| Evaluation | Emean (K) | Emin (K) | Emax (K) | Pre-exponential factor (s-1) | Order factor | Method | Origin | Reference | Type of surface | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 2400 | 0 | 0 | 0.00E+0 | 1 | Estimation | Database : OSU | H2O | This binding energy was listed in the original OSU gas-grain code from Eric Herbst group in 2006. Energy of N+N+O The pre-exponential factor is not given. It can be computed using the formula given in Hasegawa et al. (1992). |
|
Emean (K): 2400
E min (K): 0 E max (K): 0 Pre-exponential factor (s-1): 0.00E+0 Method: Estimation Origin: Other database Reference: Type of surface: H2O Description: This binding energy was listed in the original OSU gas-grain code from Eric Herbst group in 2006. Energy of N+N+O The pre-exponential factor is not given. It can be computed using the formula given in Hasegawa et al. (1992). Evaluation: |
Diffusion energy
No data