Help
- What is KIDA ?
- What type of data can I find in KIDA?
- What can you do in KIDA?
- Who is working on KIDA?
- How can I submit data to KIDA?
- I have submitted data to KIDA but I would like to modify it, how can I do?
- How can I notify KIDA that there is a mistake in some data?
- What are the quality indicators of the rate coefficients?
- What are the methods mentioned for the data?
- What are the different possible origins for the data?
- Can I add species not present in KIDA yet?
- Are data erased from KIDA?
- Can several rate coefficients appear for the same channel?
- Where can I find the references for data stored in KIDA?
- How should I reference to the database?
- How are rate coefficient uncertainties defined in KIDA?
- What are the kida.uva.xxx chemical networks?
- What are the units used in KIDA?
- What are the types of reactions?
- Which formula are used to compute the rate coefficients (for gas-phase reactions) from the parameters stored in the database?
- What are the photorates in KIDA?
- Where do the formula for unmeasured ion-polar reactions come from?
- Explanations on 3-body reactions
- Explanations on surface reactions
- How to define the surface types?
- Links and documents about chemical processes
For the interstellar medium, we provide chemical reactions occuring the gas-phase but also some parameters used to compute the interaction of species with the surface of interstellar grains and the chemical reactions occuring at the surface of these grains. We refer to Wakelam et al. (2010) and reference therein for a description of these processes. The types of data that are stored in KIDA are described here.
In KIDA, you can find different values for the same parameters from different bibliographic sources. In some cases, a quality indicator is also present. In addition to these reactions several chemical networks (a selection of reactions) specific for some astronomical objects are available. They can be directly from KIDA or compiled by users.
The KIDA database has been presented in the following scientific paper:
Wakelam et al. The Astrophysical Journal Supplement, Volume 199, Issue 1, article id. 21, 10 pp. (2012)
- Gas-phase reactions and associated partial rate coefficients (with detailed information)
- Polarizability, dipole moment and enthalpy of formation for some species.
- Desorption energies (from different types of surfaces) and diffusion energies (on different types of surfaces) for some species.
- Chemical reactions occuring at the surface of interstellar grains with branching ratios, activation energies and barrier width.
- You can find all existing data useful for the chemical modeling of interstellar chemistry and planetary atmospheres with detailed information.
- You can add data (reactions, rate coefficients, desorption energy, diffusion energy) to the database through csv templates.
- You can download a complete set of reaction rate coefficients and/or store your list of reactions.
- You can find information on the quality of the data and recommanded data for some of them.
The experts in physics and chemistry who are working on the data present in KIDA are listed here.
If you have any comments or bug reports, please send an email to kida-obsu-bordeaux.fr.
- Not recommended value
- Not rated value
- Valid value
- Recommended value
- Measurement : this means that the data has been obtained experimentally.
- Theoretical : this means that the data has been theoretically computed.
- Review and evaluation : this means that the value is the result of a compilation of published data.
- Estimation : this means that it is a guess. This can be an educated guess though.
- Bibliographic : this means that the data was extracted from a bibliographic reference (paper, thesis, book) and the reference is provided.
- Other database : this means that the data was found in another database (usually without any bibliographic reference) and the name of the database is provided.
- Datasheet : this is for recommended values.
- kida.uva.2011 (Wakelam et al. The Astrophysical Journal Supplement, Volume 199, Issue 1, article id. 21, 10 pp. (2012))
- kida.uva.2014 (Wakelam et al. The Astrophysical Journal Supplement Series, Volume 217, Issue 2, article id. 20, 7 pp. (2015))
Temperature: K
Dipole Moment: Debye
Rate coefficients for photo and cosmic-ray processes: s-1
Rate coefficients for bimolecular reactions: cm3s-1
Rate coefficients for termolecular reactions: cm6s-1
Polarizability: Å3
Enthalpy of formation and reaction: kJ mol-1
Desorption energy: Kelvin
Diffusion energy: Kelvin
activation energy: Kelvin
Barrier Width (for surface reactions): Angstrom
- Unimolecular reactions include dissociations and ionizations by cosmic-ray particules, secondary UV photons induced by cosmic-ray particles and direct UV photons. ITYPES 1 to 3.
- Bimolecular reactions includes all chemical reactions between two species. ITYPES 4 to 8
- Termolecular reactions are 3-body asssisted reactions.
- Surface reactions are reactions occuring at the surface of interstellar grains between adsorbed species.
| ITYPE | Description |
|---|---|
| 1 | Dissociation or ionization of species due to direct collision with cosmic-ray particles. |
| 2 | Dissociation or ionization of species due to UV photons emitted following H2 excitation. |
| 3 | Dissociation or ionization of neutral species by UV photons with a standard interstellar UV field. |
| 4 | Neutral-neutral (A + B → C + D), ion-neutral (A+ + B → C+ +D, A- + B → C- + D), anion-cation (A+ + B- → C + D) reactions and associative ionization (A + B → AB+ + e-) |
| 5 | Exchange reaction A+ + B → A + B+ and A+ + B- → A + B |
| 6 | Association reactions between two species (neutral or ionized) stabilized by the emission of a photon (A + B → AB + photon or A+ + B → AB+ + photon). |
| 7 | Association of a neutral species and an anion, resulting in the ejection of the extra electron (A- + B → AB + e-). |
| 8 | Recombination of a positive ion with an electron resulting in the dissociation of the molecule (AB+ + e- → A + B) or the emission of a photon (AB+ + e- → AB + photon) or the attachment of the electron (A + e- → A- + photon) |
| Number (for export) | Name | Formula | Units |
|---|---|---|---|
| 1 | Cosmic-ray ionization | 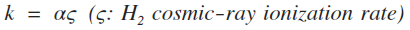 |
s-1 |
| 2 | Photo-dissociation (Draine) | 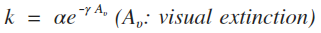 |
s-1 |
| 3 | Modified Arrhenius | 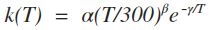 |
cm3 s-1 |
| 4 | ionpol1 | 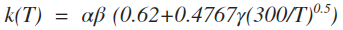 |
cm3 s-1 |
| 5 | ionpol2 | 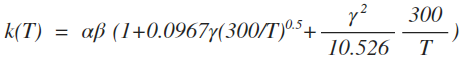 |
cm3 s-1 |
| 6 | 3-body | See here | |
For information on the visual extinction Av see here.
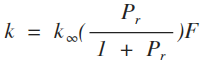
The broadening factor F is the factor by which the rate constant of a given recombination reaction at temperature T and at the reduced pressure Pr is reduced from the value it would have if recombination reactions behaved according to the Lindemann formula.
The reduced pressure Pr is compted by Pr =k0[M]/kinf with [M] the concentration of the third body.
k0 and k∞ are function of temperature computed with the Kooij formula. k0 is the low-pressure limiting form whereas kinf is the high-pressure limit. Both rate constants have the following unit: cm6.molecules-2.s-1.
The broadening factor F for every reduced pressures Pr is computed as:
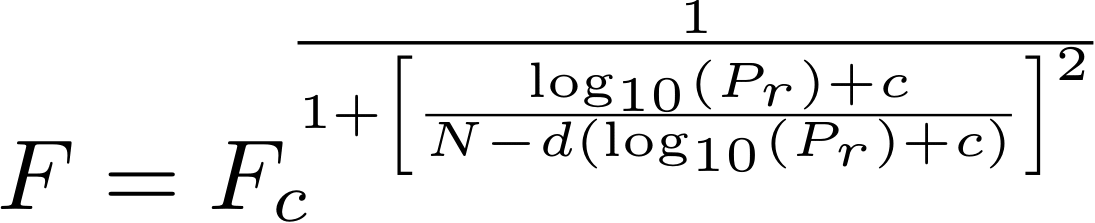
c = - 0.4 - 0.67 x log10(Fc)
N = 0.75 - 1.27 x log10(Fc)
d = 0.14
In some cases, Fc can be calculated as a function of temperature from the Troe's fall-off parameters a, b, c, and d by:
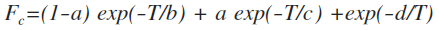
a is dimensionless and b, c and d are in K (T as well of course).
When no specific Troe fall-off parameters can be retrieved from the literature, it can be assumed Fc = 0.64, following the simpler policy chosen by the NASA/JPL evaluation panel (Sander et al., 2006).
In KIDA, you will find Fc and the parameters to compute k0 and kinf.
In KIDA, we store 5 types of data for surface reactions: Two data specific to species:
- Binding energies
- Diffusion energies
For reactions, we store:
- branching ratios
- activation energies
- barrier width for surface reactions with activation energies to compute the rate coefficient with quantum tunneling (following the formula given in eq (6) of Hasegawa et al. 1992 http://articles.adsabs.harvard.edu/pdf/1992ApJS...82..167H for a rectangular barrier)
Concerning parameters for the surface reactions and gas-grain interactions, the available data is very sparse. So as a start, we have put in the database what we have been using in our models. A BIG work on quality data have just started.
- Photo-processes: Many information about the computation of the rates of photo-reactions can be found in Ewine van Dishoeck's web page at the University of Leiden (Netherlands): here.
- The ISSI Team: To improve our knowledge on the kinetic of cold interstellar regions, we made a group of both astrophysicists and physico-chemists. Two meeting of this group in Bern (Switzerland) were funded in 2008 by the International Space Science Institute (http://www.issibern.ch/). The results of our discussions have been written in an article submitted to Space Science Reviews and can be downloaded here.
- The UMIST Database for Astrochemistry (http://udfa.ajmarkwick.net/)
- The NIST webbook (http://webbook.nist.gov/chemistry/form-ser.html)